alb10703592
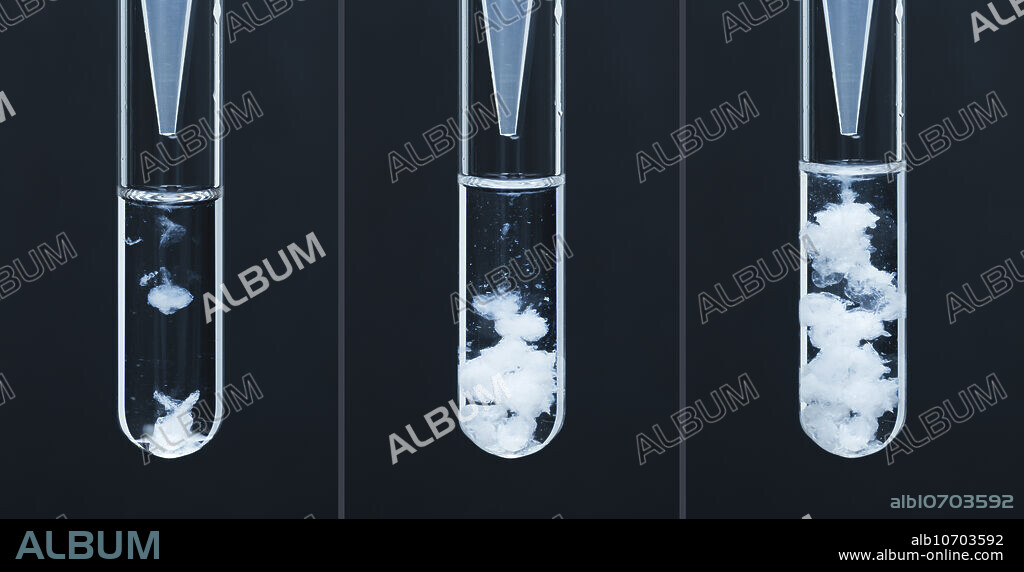
Barium carbonate precipitate
|
Zu einem anderen Lightbox hinzufügen |
|
Zu einem anderen Lightbox hinzufügen |



Haben Sie bereits ein Konto? Anmelden
Sie haben kein Konto? Registrieren
Dieses Bild kaufen.
Nutzung auswählen:

Titel:
Barium carbonate precipitate
Untertitel:
Siehe automatische Übersetzung
Barium carbonate precipitate. Barium carbonate (BaCO3) precipitate formed by adding barium chloride solution (BaCl2) drop by drop to sodium carbonate solution (Na2CO3). Both solutions are 0.5 M concentration. The reaction is Na2CO3 + BaCl2 -> BaCO3 + NaCl. This is an example of a double replacement reaction.
Bildnachweis:
Album / Science Source / GIPhotoStock
Freigaben (Releases):
Model: Nein - Eigentum: Nein
Rechtefragen?
Rechtefragen?
Bildgröße:
7000 x 3549 px | 71.1 MB
Druckgröße:
59.3 x 30.0 cm | 23.3 x 11.8 in (300 dpi)
Schlüsselwörter:
 Pinterest
Pinterest Twitter
Twitter Facebook
Facebook Link kopieren
Link kopieren Email
Email
