alb3800502
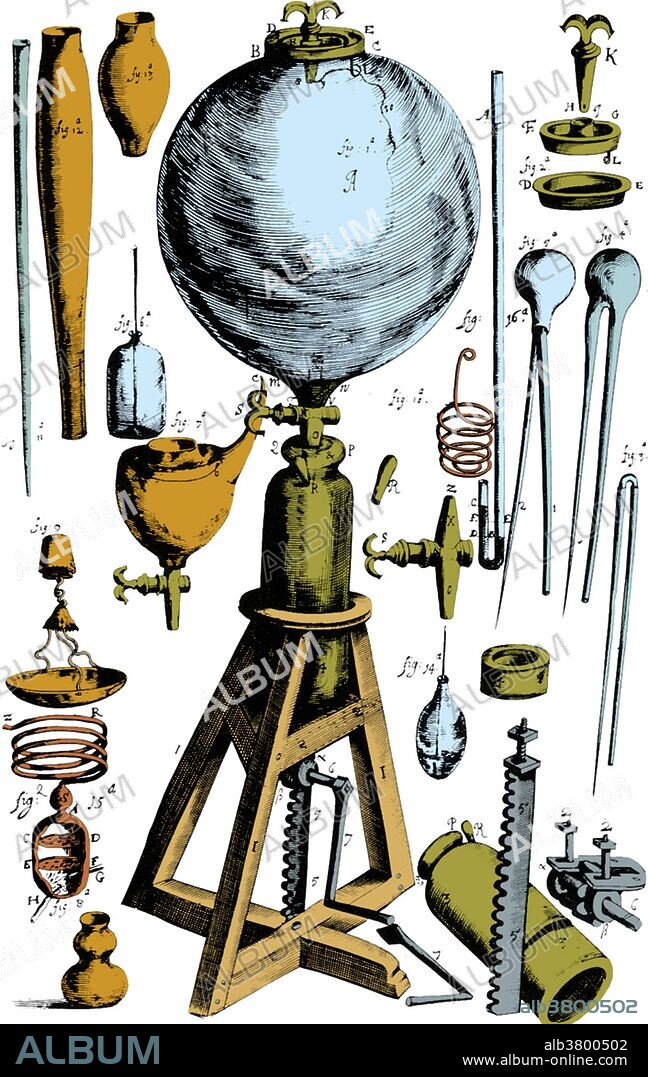
Robert Boyle, Experimental Air Pump
|
Zu einem anderen Lightbox hinzufügen |
|
Zu einem anderen Lightbox hinzufügen |



Haben Sie bereits ein Konto? Anmelden
Sie haben kein Konto? Registrieren
Dieses Bild kaufen.
Nutzung auswählen:

Titel:
Robert Boyle, Experimental Air Pump
Untertitel:
Siehe automatische Übersetzung
Among Boyle's earliest scientific work were studies involving the air pump. At the time, Robert Hooke was Boyle's laboratory assistant. Starting with the German physicist Otto von Guericke's description of an air pump, Hooke improved on its design, reducing its size and increasing its performance while making it easier to use. Utilizing this improved air pump, Boyle devised experiments to explore the properties of air. He examined the behavior of sound, heat, light, electricity, magnetism, chemical reactions (such as a flame), and living systems (such as small animals or plants) in a vacuum. He also considered the behavior of the air itself under extension or compression. The result of this study was the relationship now known as Boyle's law, which states that the pressure and volume of a confined air (gas) are inversely related. Mathematically, this is expressed as pressure times volume equals a constant: PV = constant.
Bildnachweis:
Album / Science Source
Freigaben (Releases):
Model: Nein - Eigentum: Nein
Rechtefragen?
Rechtefragen?
Bildgröße:
4200 x 6639 px | 79.8 MB
Druckgröße:
35.6 x 56.2 cm | 14.0 x 22.1 in (300 dpi)
Schlüsselwörter:
ALCHEMIE • BERÜHMT • BERÜHMTE PERSÖNLICHKEIT • CHEMIE • CHEMIKER • ERFINDER • EUROPAEER (F M) • EUROPAEER • EUROPÄER (F M) • EUROPÄER • EUROPÄISCH • ILLUSTRATION • ILLUSTRATIONS • IRISCH • LUFTPUMPE • NATURPHILOSOPH • NOTABEL • PROMINENZ • WISSENSCH.: CHEMIE
 Pinterest
Pinterest Twitter
Twitter Facebook
Facebook Link kopieren
Link kopieren Email
Email
