alb3796261
Electrons orbiting atom
|
Add to another lightbox |
|
Add to another lightbox |



Title:
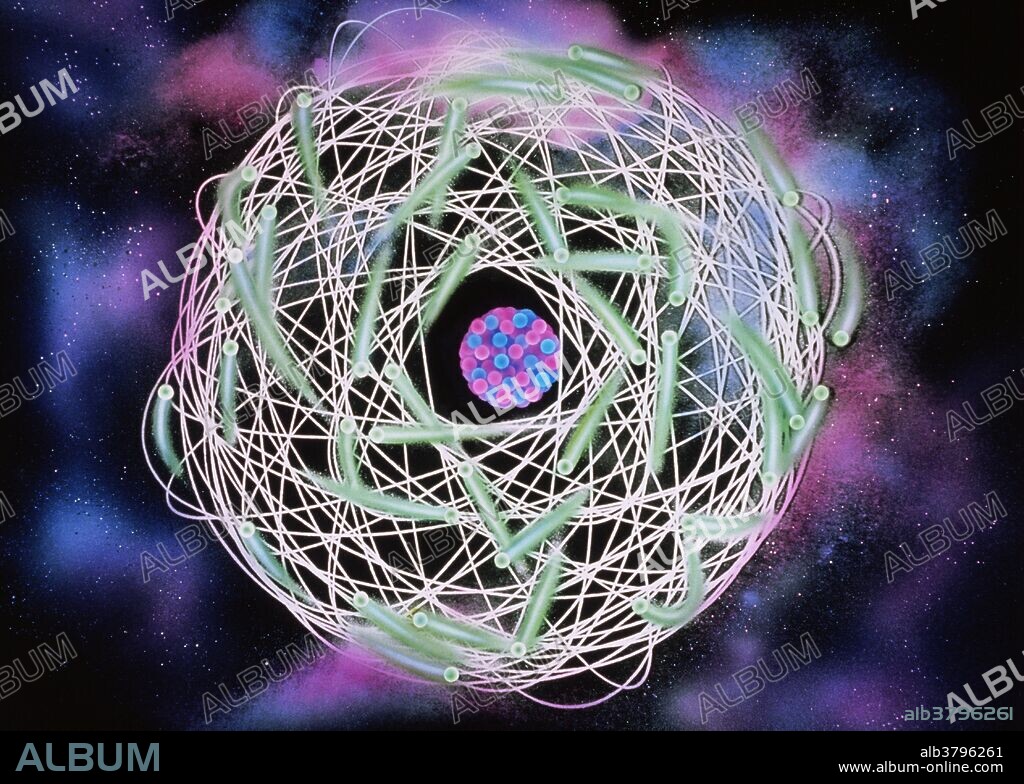
Electrons orbiting atom
Caption:
Artist's impression of electron orbitals surrounding an atomic nucleus. This representation owes much to classical physics, and shows the electrons as discrete particles inhabiting fixed orbital paths. This is known as the Bohr Model. Quantum physics describes the electrons as inhabiting a probability 'cloud' around the nucleus, and exhibiting both particle and wave properties. A classical fixed orbit would allow the precise measurement of position and velocity of a given electron, prohibited by Heisenberg's Uncertainty Principle in the quantum world.
Credit:
Album / Science Source / Omikron
Releases:
Model: No - Property: No
Rights questions?
Rights questions?
Image size:
4486 x 3202 px | 41.1 MB
Print size:
38.0 x 27.1 cm | 15.0 x 10.7 in (300 dpi)
Keywords:
ART • ARTWORK • ATOM ATOM NUCLEAR • ATOM • ATOMIC CHEMISTRY • ATOMIC MODEL • ATOMIC NUCLEUS • ATOMIC PARTICLE • ATOMIC PARTICLES • ATOMIC PHYSICS • ATOMIC STRUCTURE • ATOMO • ATOMS • BIOLOGY • C. G. • CHEMISTRY • COMPOUND • COMPUTER MODEL • CORE • ELECTRON • ELECTRONS • ELEMENT • ÉLÉMENTS • HI TECH • HI-TECH • HIGH TECH • HIGH TECHNOLOGY • HIGH-TECH • ILLUSTRATION • ILLUSTRATIONS • MODEL • MOLECULAR BIOLOGY PARTICLE • MOLECULAR CHEMISTRY • MOLECULAR GRAPHICS • MOLECULAR MODEL • MOLECULAR MODELS • MOLECULAR PHYSICS • MOLECULAR PROPERTIES • MOLECULAR STRUCTURE • MOLECULAR STRUCTURES • MOLÉCULE • MOLECULES • NEUTRON • NEUTRONS • NUCLEUS • ORBIT • ORBITAL • ORBITING • ORBITS • P ORBITAL • PARTICLE • PARTICLES • PHYSICS • PROTON • PROTONS • QUARK • QUARKS • RENDER • RENDERING • RESEARCH • S ORBITAL • SCIENCE • VERTICAL LINES • VERTICAL
 Pinterest
Pinterest Twitter
Twitter Facebook
Facebook Copy link
Copy link Email
Email

