alb3801363
Bunsen Cell Battery, 19th Century
|
Add to another lightbox |
|
Add to another lightbox |



Buy this image.
Select the use:

Title:
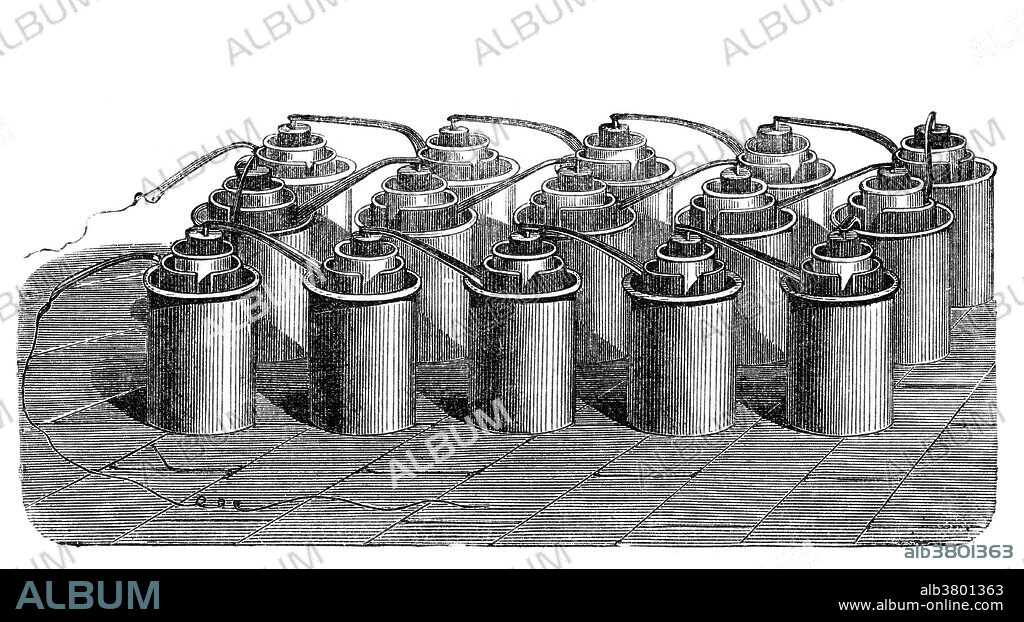
Bunsen Cell Battery, 19th Century
Caption:
The Bunsen cell battery is a zinc-carbon primary cell composed of a zinc anode in dilute sulfuric acid separated by a porous pot from a carbon cathode in nitric or chromic acid. The cell is named after its inventor, German chemist Robert Wilhelm Bunsen, who improved upon the Grove cell by replacing Grove's expensive platinum cathode with carbon in the form of pulverized coal and coke. Like Grove's battery, Bunsen's emitted noxious fumes of nitrogen dioxide. Bunsen used this cell to extract metals. Henri Moissan used a stack of 90 cells for the electrolysis of hydrogen fluoride to obtain fluorine for the first time.
Credit:
Album / Science Source
Releases:
Model: No - Property: No
Rights questions?
Rights questions?
Image size:
4800 x 2665 px | 36.6 MB
Print size:
40.6 x 22.6 cm | 16.0 x 8.9 in (300 dpi)
Keywords:
19TH CENTURY • ART • ARTWORK • BATTERY • BUNSEN BATTERY • BUNSEN CELL BATTERY • BUNSEN CELL • BUNSEN PILE • BUNSEN • BW • CELEBRITIES • CELEBRITY • DRAWING • ELECTRIC • ELECTRICAL • ELECTRICITY • ELECTROSTATICS • ENGRAVING • FAMOUS PEOPLE • FAMOUS • FIGUIER • GREAT INVENTIONS • HISTORIC • HISTORICAL • HISTORY • ILLUSTRATION • ILLUSTRATIONS • IMPORTANT • INVENTION • LES GRANDES INVENTIONS • LOUIS FIGUIER • NOTABLE • PHYSICS • PRIMARY CELL • ROBERT BUNSEN • ROBERT WILHELM EBERHARD BUNSEN • SCIENCE • STATIC ELECTRICITY • WELL-KNOWN • ZINC-CARBON
 Pinterest
Pinterest Twitter
Twitter Facebook
Facebook Copy link
Copy link Email
Email
