alb3800502
Robert Boyle, Experimental Air Pump
|
Add to another lightbox |
|
Add to another lightbox |



Buy this image.
Select the use:

Title:
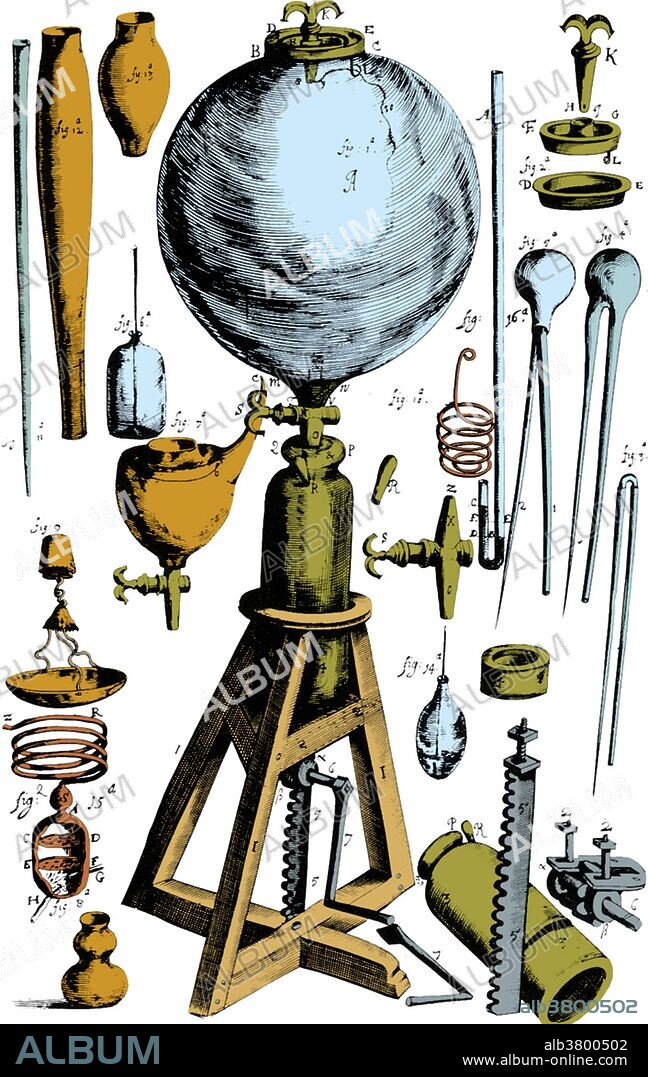
Robert Boyle, Experimental Air Pump
Caption:
Among Boyle's earliest scientific work were studies involving the air pump. At the time, Robert Hooke was Boyle's laboratory assistant. Starting with the German physicist Otto von Guericke's description of an air pump, Hooke improved on its design, reducing its size and increasing its performance while making it easier to use. Utilizing this improved air pump, Boyle devised experiments to explore the properties of air. He examined the behavior of sound, heat, light, electricity, magnetism, chemical reactions (such as a flame), and living systems (such as small animals or plants) in a vacuum. He also considered the behavior of the air itself under extension or compression. The result of this study was the relationship now known as Boyle's law, which states that the pressure and volume of a confined air (gas) are inversely related. Mathematically, this is expressed as pressure times volume equals a constant: PV = constant.
Credit:
Album / Science Source
Releases:
Model: No - Property: No
Rights questions?
Rights questions?
Image size:
4200 x 6639 px | 79.8 MB
Print size:
35.6 x 56.2 cm | 14.0 x 22.1 in (300 dpi)
Keywords:
1627 • 1691 • 17TH CENTURY • AIR PUMP • ALCHEMIST • ALCHEMY • ALCHEMY, ALCHEMIST • ART • ARTWORK • AUTHOR • BOYLE • CELEBRITIES • CELEBRITY • CHEMIST • CHEMISTRY • COLORIZED • DRAWING • ENHANCEMENT • EUROPEA • EUROPEAN • EUROPEANS • EXPERIMENTAL • FAMOUS PEOPLE • FAMOUS • HISTORIC • HISTORICAL • HISTORY • ILLUSTRATION • ILLUSTRATIONS • IMPORTANT • INFLUENTIAL • INVENETION • INVENTOR (MALE) • INVENTOR • IRISH • MODERN CHEMIST • NATURAL PHILOSOPHER • NOTABLE • PHYSICIST • PHYSICS • POLYMATH • ROBERT BOYLE • SCIENCE • SCIENTIFIC METHOD • THEOLOGIAN • WELL-KNOWN • WRITER
 Pinterest
Pinterest Twitter
Twitter Facebook
Facebook Copy link
Copy link Email
Email